Areas of Research
& Special Interests:


Educational &
Academic Interests:
|
|
 |
Spike Initiation in Retinal Ganglion Cells
The Achilles Heel of the Ganglion Cell?
The axons of most retinal ganglion cells emerge from the cell body with a tapering structure called the axon hillock (AH). A remarkable thinning of the axon takes place shortly after its emergence from the AH in which the axon diameter may be reduced to 0.2 - 0.4 µm diameter, with a length as long as 50 to 100 microns. The thin segment or narrow region (NR) then merges with an axon that is usually about 1 micron (tiger salamander and mudpuppy) in diameter and remains at that diameter as it courses through the optic nerve fiber layer to the optic disk. The existence of the NR raises a number of problems concerning the site and mechanism of impulse generation. As the diameter of an axon gets smaller, impulse generation and propagation become more problematic as the core resistance gets disproportionately larger. Thus, one possibility, which was considered by our laboratory (Carras, Coleman and Miller, 1992) was to imagine that the site of impulse initiation for retinal ganglion cells was downstream from the thin segment, which behaved as a passive structure and would enhance cable losses. However, whole-cell recordings from ganglion cell bodies showed that the impulse could be back propagated from stimulation of the nerve into the soma; this could only occur if the NR was capable of generating an impulse.
To illustrate how this takes place, we have structured a simulation of an antidromic impulse, by using a realistic cell model, compartmentalized and visually expanded using the Neuron simulator. In this simulation an antidromic impulse was started in the distal axon and propagated into the soma. The model used for this simulation is the five-channel model developed by Fohlmeister and Miller (Fohlmeister and Miller, 1997a & b) and the channel densities are illustrated in this table. The anatomical constraints include multiple, tapering sections from the NR to the distal axon and the proximal AH. As the impulse propagates through the NR and approaches the AH, it begins to fail, even though the Na+ channel density in the NR is relatively high. Nevertheless, at these channel densities, an impulse in the AH and soma-dendritic tree takes place. This result accurately simulates the physiological studies based on whole-cell recordings. In order for the NR to conduct nerve impulses the Na+ channel density must be relatively high and, for the orthograde impulse to function properly, the soma and the dendritic tree must contain Na+ channels. Although the actual site of impulse initiation in retinal ganglion cells has not been identified, the presence of elevated Na+ channels in the AH and NR suggests that both structures play an important role in the early stages of impulse generation and propagation towards the brain.
 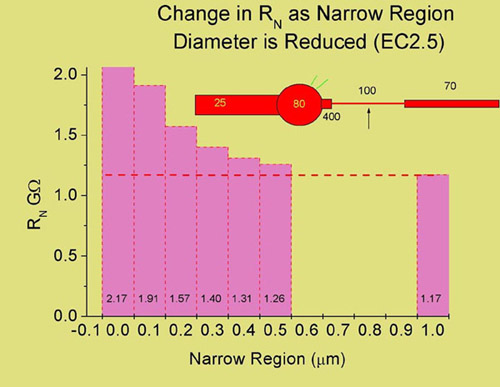
The Function of the Narrow Axonal Region
The simplified model of a ganglion cell (pictured above) consists of an equivalent cylinder dendritic tree, a soma, axon hillock, narrow region and axon. The Na+ channel densities (mS/cm2) are those used to successfully simulate an antidromic action potential. When synaptic currents are generated in the dendrites, the axon itself serves as a shunt which lowers the input resistance of the cell at the cell body. In this example, using a membrane resistance of 70,000 ohm cm2 and an internal resistance of 110 ohm cm, we calculated the input resistance at the soma when the diameter of the NR is altered. In the absence of the NR, the input resistance of the cell is 2.17 Gohms and if the NR is the same diameter as the distal axon (1 µm), the input resistance drops to 1.17 Gohms. Thus, the NR can serve to enhance synaptic efficacy by reducing the shunt that the axon presents. This is only one of several different possibilities however and a direct role in supporting impulse generation and propagation awaits further elucidation.
References
Carras, P.L., Coleman, P.A. and Miller, R.F. (1992) Site of action potential initiation in amphibian retinal ganglion cells. J. Neurophysiol. 67(2):292-304).
Fohlmeister, J.F. and Miller, R.F. (1997) Impulse encoding mechanisms of ganglion cells in the tiger salamander retina. J. Neurophysiol. 78:1935-1947.
Fohlmeister, J.F. and Miller, R.F. (1997) Mechanisms by which cell geometry controls repetitive impulse firing in retinal ganglion cells. J. Neurophysiol. 78:1948-1964
|
|
![]() Return
to: Dept. of Neuroscience : U of M Home
Return
to: Dept. of Neuroscience : U of M Home